Guideline on GCP compliance in relation to trial master file (paper and/or electronic) for content, management, archiving, audit

Good Clinical Practice Training: Identifying Key Elements and Strategies for Increasing Training Efficiency - Jaime Arango, Tina Chuck, Susan S. Ellenberg, Bridget Foltz, Colleen Gorman, Heidi Hinrichs, Susan McHale, Kunal Merchant, Jonathan

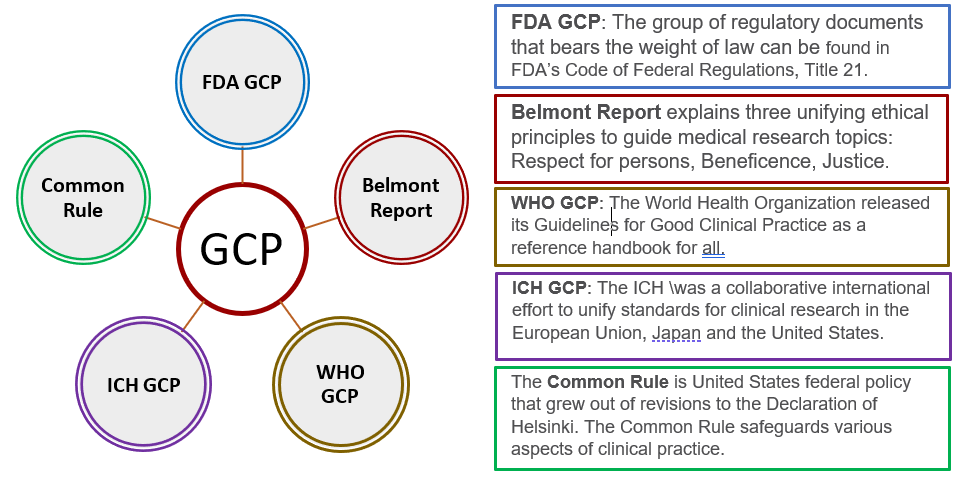
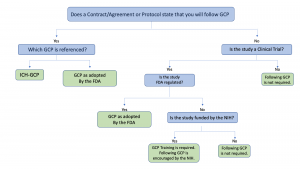
ICH GCP - 8. Essential documents for the conduct of a clinical trial: ICH E6 (R2) Good clinical practice

Drones | Free Full-Text | Object Recognition of a GCP Design in UAS Imagery Using Deep Learning and Image Processing—Proof of Concept Study
ICH GCP - 8. Essential documents for the conduct of a clinical trial: ICH E6 (R2) Good clinical practice