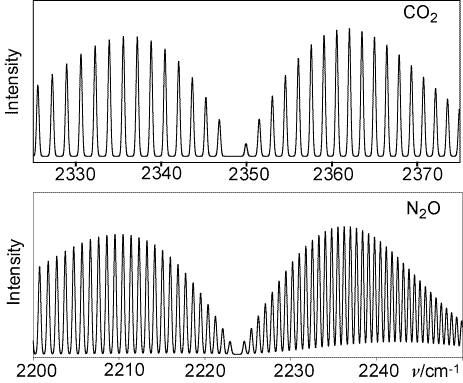
![PDF] Vibrational spectra of N2: An advanced undergraduate laboratory in atomic and molecular spectroscopy | Semantic Scholar PDF] Vibrational spectra of N2: An advanced undergraduate laboratory in atomic and molecular spectroscopy | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/9e5e7c471967f5326750e1d04ca047afcf337cf1/4-Figure2-1.png)
PDF] Vibrational spectra of N2: An advanced undergraduate laboratory in atomic and molecular spectroscopy | Semantic Scholar

Cyanamide as an Infrared Reporter: Comparison of Vibrational Properties between Nitriles Bonded to N and C Atoms | The Journal of Physical Chemistry B
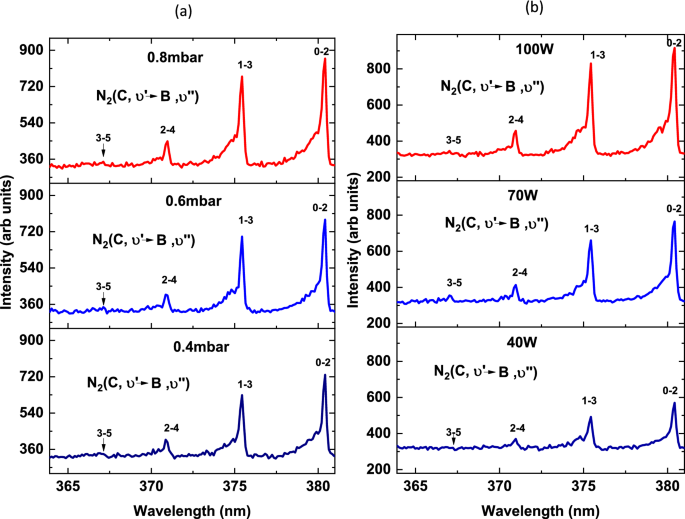
Spectroscopic evaluation of vibrational temperature and electron density in reduced pressure radio frequency nitrogen plasma | SpringerLink

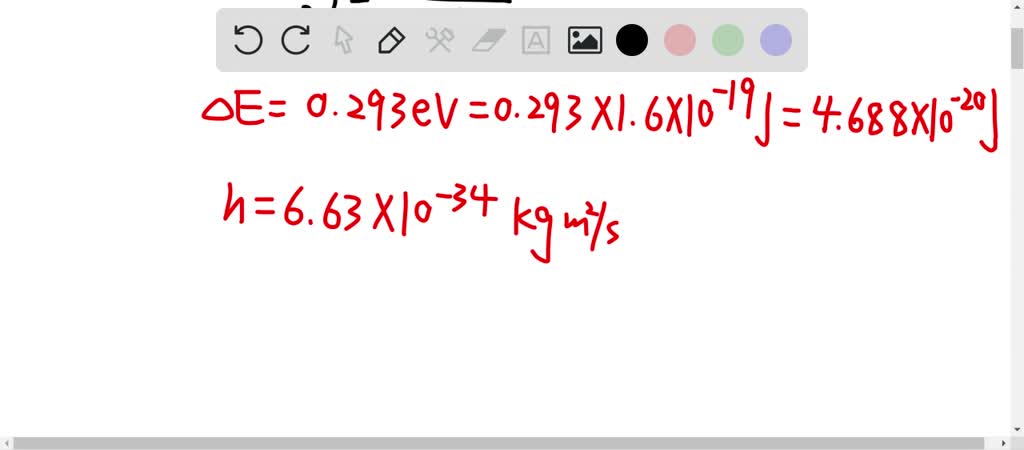
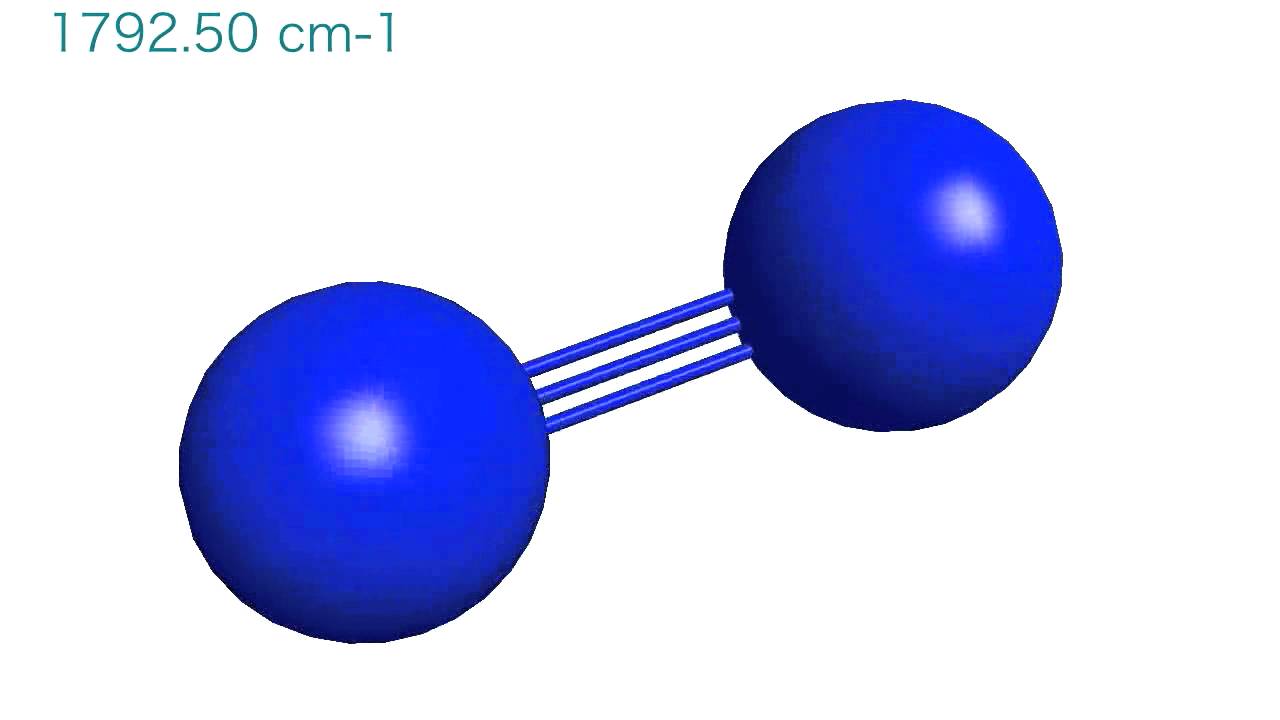
The natural vibration frequency of a hydrogen molecule is 1.26xx10^(14) Hz. Find the zero-point energy of the vibrations of the molecule. Can the vibrational degrees of freedom in the molecule be excited
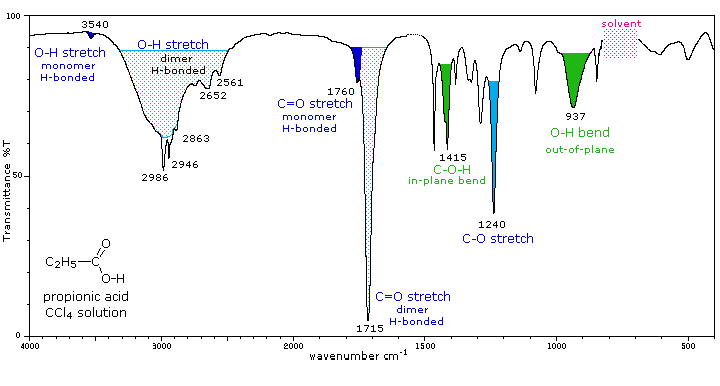
The Spectrum of Nitrogen Dioxide in the 1.4–3.4μ Region and the Vibrational and Rotational Constants of the NO2 Molecule*

Determination of vibrational and rotational temperatures in highly constricted nitrogen plasmas by fitting the second positive system of N2 molecules: AIP Advances: Vol 5, No 5

FT-IR spectra showing stretching vibrations of metal to nitrogen in... | Download Scientific Diagram
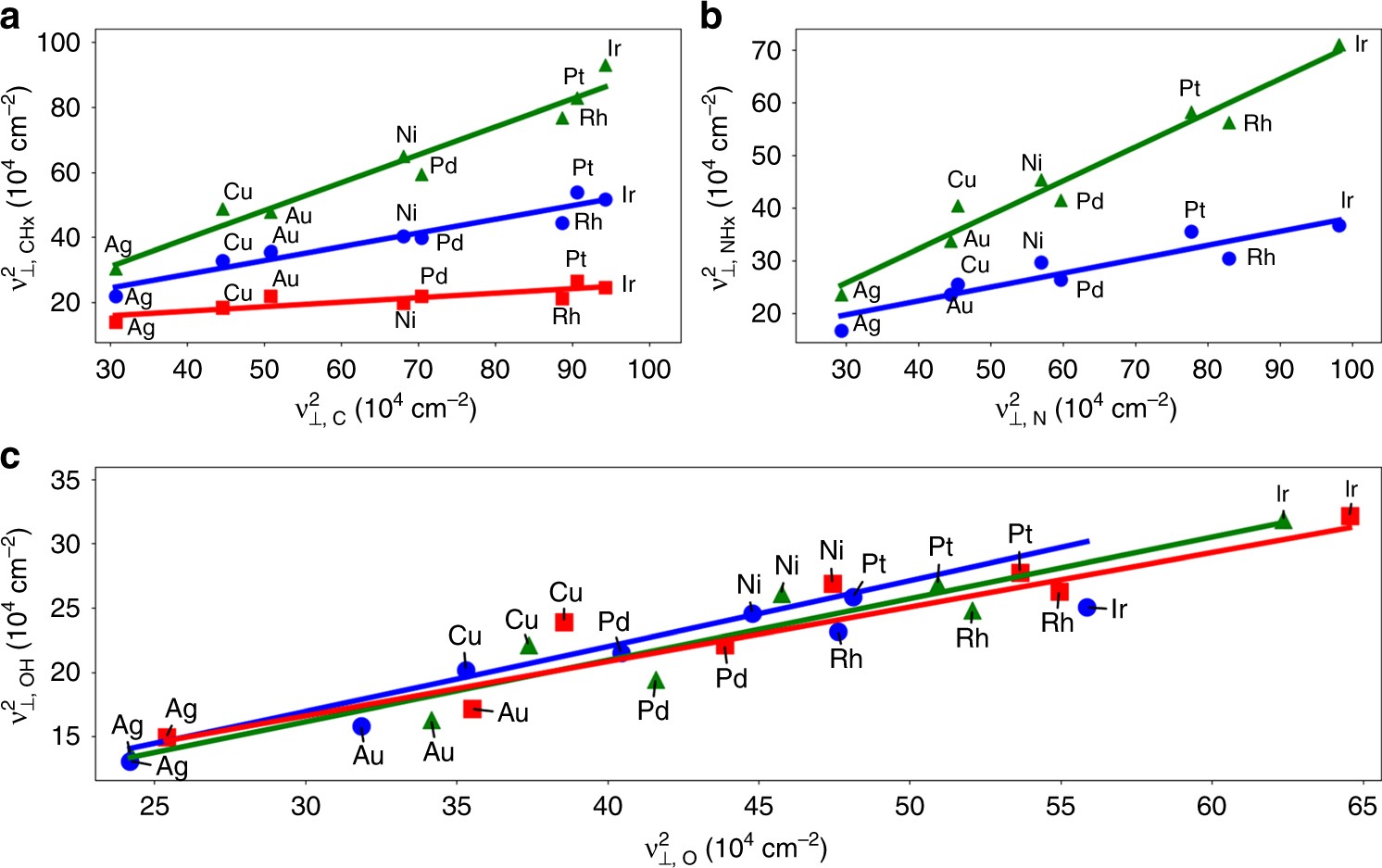
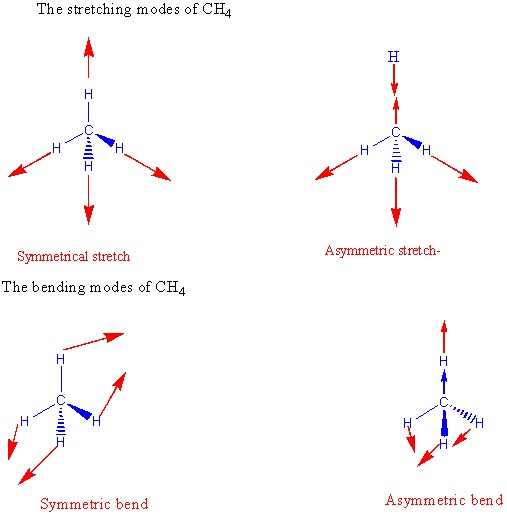
Scaling relationships and theory for vibrational frequencies of adsorbates on transition metal surfaces | Nature Communications

Determination of vibrational and rotational temperatures in highly constricted nitrogen plasmas by fitting the second positive system of N2 molecules: AIP Advances: Vol 5, No 5
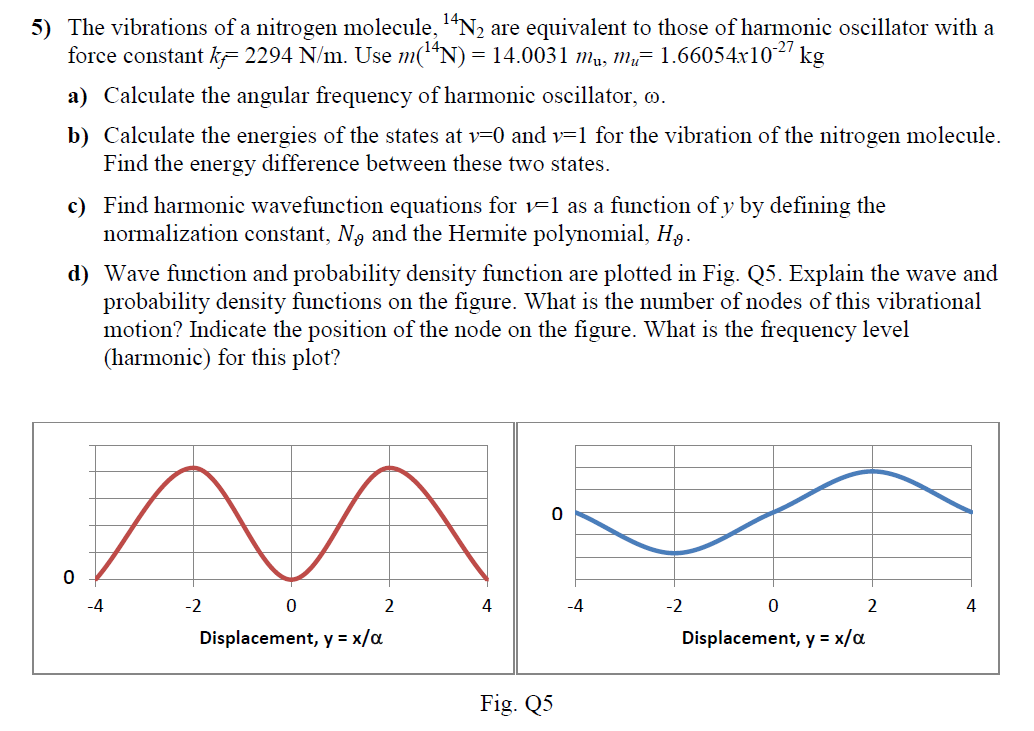
A. Given the force constants for N2 and O2 are 2287 and 1133 N/m, respectively, calculate their vibrational frequencies. Solutio